
Sulforaphane Inhibits NLRP3 Inflammasome in Murine Microglial Cells
Sulforaphane is a potent inhibitor of NLRP3
The SARS (severe acute respiratory syndrome) outbreak was caused by a coronavirus (CoV) named the SARS-CoV.
SARS pathology is propagated both by direct cytotoxic effects of the virus and aberrant activation of the innate immune response.
Here, we identify several mechanisms by which a SARS-CoV open reading frame (ORF) activates intracellular stress pathways and targets the innate immune response. We show that ORF8b forms insoluble intracellular aggregates dependent on a valine at residue 77.
Aggregated ORF8b induces endoplasmic reticulum (ER) stress, lysosomal damage, and subsequent activation of the master regulator of the autophagy and lysosome machinery, Transcription factor EB (TFEB). ORF8b causes cell death in epithelial cells, which is partially rescued by reducing its ability to aggregate. In macrophages, ORF8b robustly activates the NLRP3 inflammasome by providing a potent signal 2 required for activation.
Mechanistically, ORF8b interacts directly with the Leucine Rich Repeat domain of NLRP3 and localizes with NLRP3 and ASC in cytosolic dot-like structures. ORF8b triggers cell death consistent with pyroptotic cell death in macrophages.
While in those cells lacking NLRP3, accumulating ORF8b cytosolic aggregates cause ER stress, mitochondrial dysfunction, and caspase-independent cell death.
The NLRP3 inflammasome is a caspase-1-containing multi-protein complex that controls the release of IL-1β and plays important roles in the development of inflammatory disease.
Introduction:
Sulforaphane (1-Isothiocyanato-4-methylsulfinylbutane) is a naturally occurring compound in cruciferous vegetables and is organo-sulfur compound that has isothiocyanate group [1].
Sulforaphane is a phytochemicals which are capable of illustrating anti-inflammatory effects is good candidate to inhibit inflammasome activation.
Main inflammatory glial cell type in the central nervous system (CNS) is microglia [2]. NACHT, LRR and PYD domains containing protein 3 (NALP3) inflammasome is encoded by NLRP3, NOD-like receptor family, gene. These large multiprotein complex plays role in innate immunity by initiating microglial activation which triggers inflammatory responses that IL-1β and IL-18 production controlled by caspase-1 containing multi-protein complex [3].
NLRP3 inflammasome is activated by large number of stimuli like metabolic stress products (cholesterol crystals, ATP, monosodium urate crystals etc.) and exogenous molecules (asbestos, silica etc.) differently rather than the canonical pathways [4]. Pyroptotic cell death may also be caused by inflammasome activation. Therefore, it should be inhibited under such conditions.
The view of the present study is to evaluate the effects of sulforaphane on NLRP3 inflammasome activation. We found that sulforaphane decreased the IL-1β and IL-18 cytokine level and cell death caused by LPS-induced inflammasome activation in murine microglial cells.
Methods:
Sulforaphane (SFN) and adenosine 5’-triphosphate (ATP) disodium hydrate were obtained from Sigma-Aldrich (St. Louis, USA) and Lipopolysaccharides (LPS, 0111:B4) were purchased from InvivoGen (San Diego, USA). N9 microglial cells provided by Dr. Paola Ricciardi-Castagnoli (Cellular Pharmacology Center, Milan, Italy) were cultured in RPMI 1640 supplemented with 2mM LGlutamine, 10% Fetal bovine serum (FBS), 1% penicillin/streptomycin (100 U/ml penicillin and 100 μg/ml streptomycin) at 37°C in an incubator containing 5% CO2.
Microglial cells were pretreated with sulforaphane (5 μM) for 1 hour followed by LPS treatment for 4 hours and ATP treatment for 1 hour. Cells viability was performed using a Cell-Counting Kit-8 (Sigma Aldrich, USA). To detect and quantify of cell-mediated cytotoxicity, Cytotoxicity Detection Kit LDH (Roche, Germany) was used. Cellular caspase-1 activity was measured by Caspase-1/ICE Colorimetric Assay Kit (R&D Systems, Minneapolis, MN, USA). For ELISA, Cell culture supernatants were collected before centrifugation to remove insoluble material. Levels of secreted IL-1β and IL-18 in cell culture supernatants were measured respectively using Mouse IL-1beta Platinum ELISA (eBioscience, USA) and Mouse IL-18 ELISA Kit (MBL International, Japan).
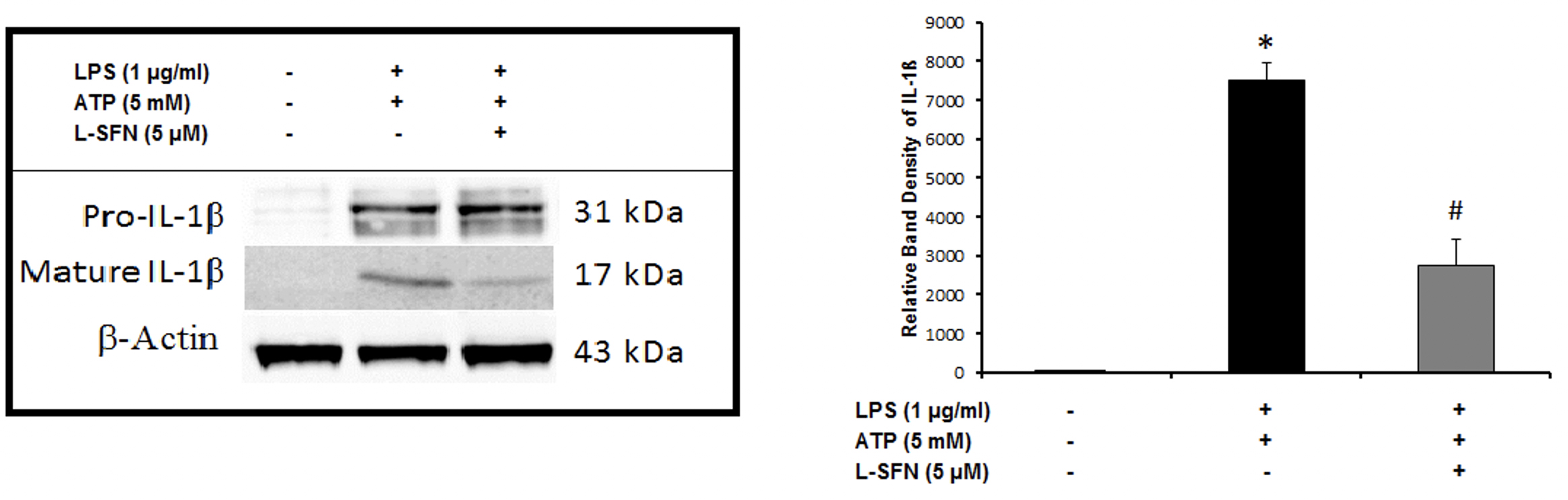
For Western-blotting, total cellular proteins were isolated. Cytosolic proteins were extracted using methanol/chloroform precipitation. Equal amounts of supernatant and lysate samples were separated by SDS-PAGE and blotted. The membranes were blocked with 5% non-fat dry milk or BSA in PBS-T (0.2% Tween-20 in 1 xPBS, pH 7.2) for 1 h and probed overnight at 4ºC with anti-mouse IL-1β antibody (Ab9722, Abcam, CA, USA) followed by incubation with HRP-conjugated secondary Abs for 1h. Protein bands were normalized to β-actin.
For real-time PCR, total RNA was extracted from N9 microglial cells using a total RNA kit (M&N, Germany). cDNA samples were amplified using the mouse primers for IL-1β, IL-18 and NLRP3 target genes. In all assays, standard program was used for cDNA amplification (10 min initial denaturation at 95ºC, 45 amplification cycles of 10s at 95 ºC, annealing of 10s at 60ºC, and extension of 20s at 72 º C). Quantitative real-time PCR products were analyzed using melting curve analysis and relative gene expression levels were measured by using the 2-(ΔΔCT) method. Glyceraldehyde-3- phosphate dehydrogenase (GAPDH) was used as the housekeeping gene to normalize gene expression in each sample.
For each experiment, SPSS 19.0 software (IBM, USA) was used for data analysis in triplicate and presented as means ± S.E.M. Mean comparisons between sulforaphane-treated cells and untreated control cells were analyzed using Mann-Whitney U test. P values <0.05 were considered as statistically significant.
Results:
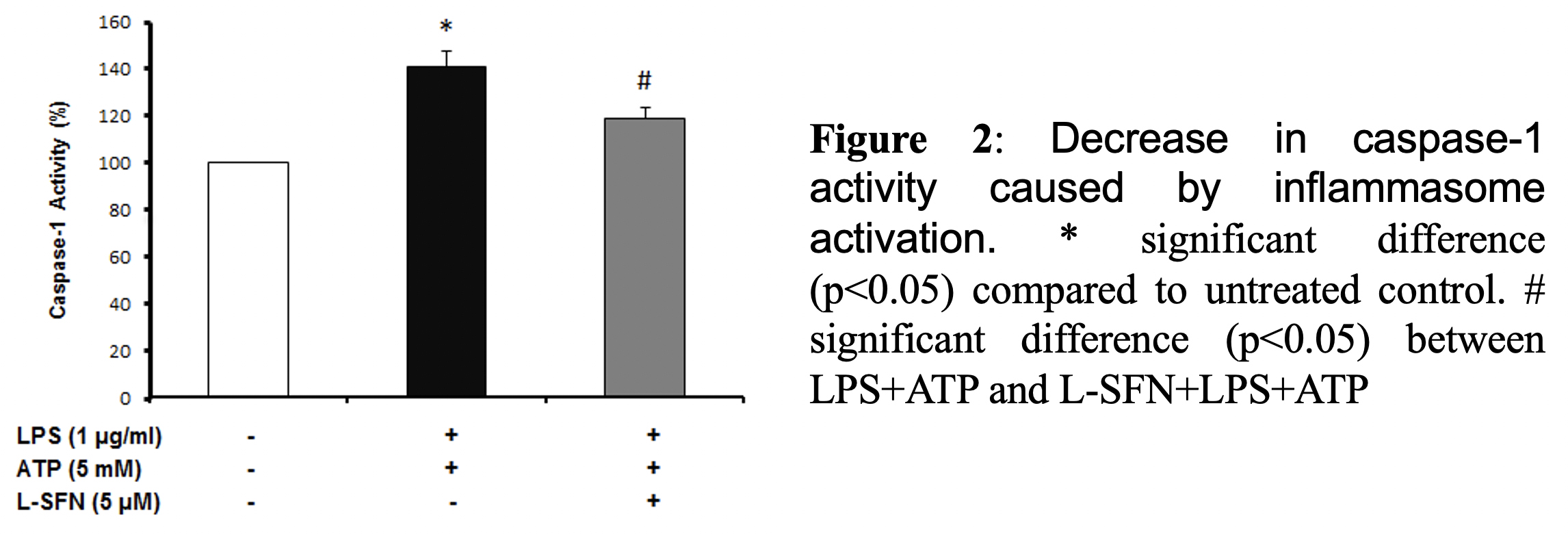
As a result of cell viability and cell cytotoxicity tests, natural form of sulforaphane illustrated neuroprotective effect on N9 microglial cells from ATP induced inflammasome dependent cell death (Figure 1). We asked if sulforaphane attenuates caspase-1 activity upon inflammasome activation by LPS and ATP. Pretreatment of SFN reduced caspase-1 activity compared to LPS+ATP group (Figure 2).
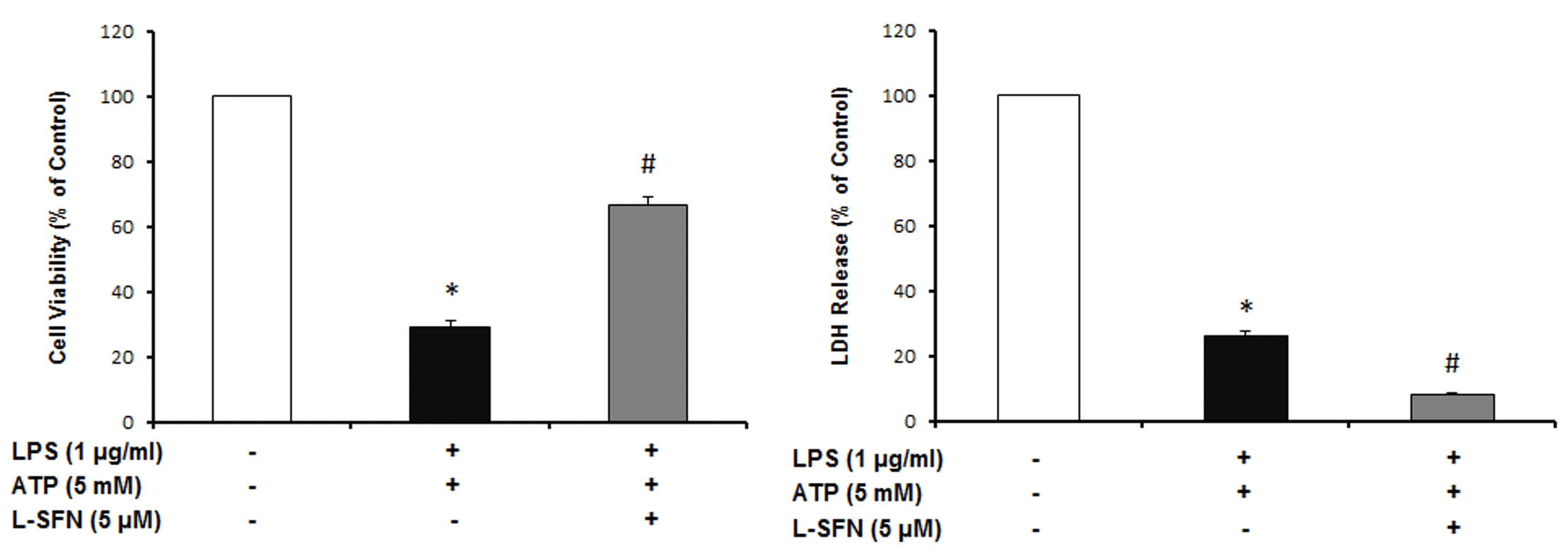
Figure 1: L-SFN protected N9 microglial cells from inflammasome dependent cell death. * significant difference (p<0.05) compared to untreated control. #
We investigated whether SFN attenuates pro-inflammatory cytokines (IL-18, IL-1ß) secreted by inflammasome activation. After caspase-1 activation
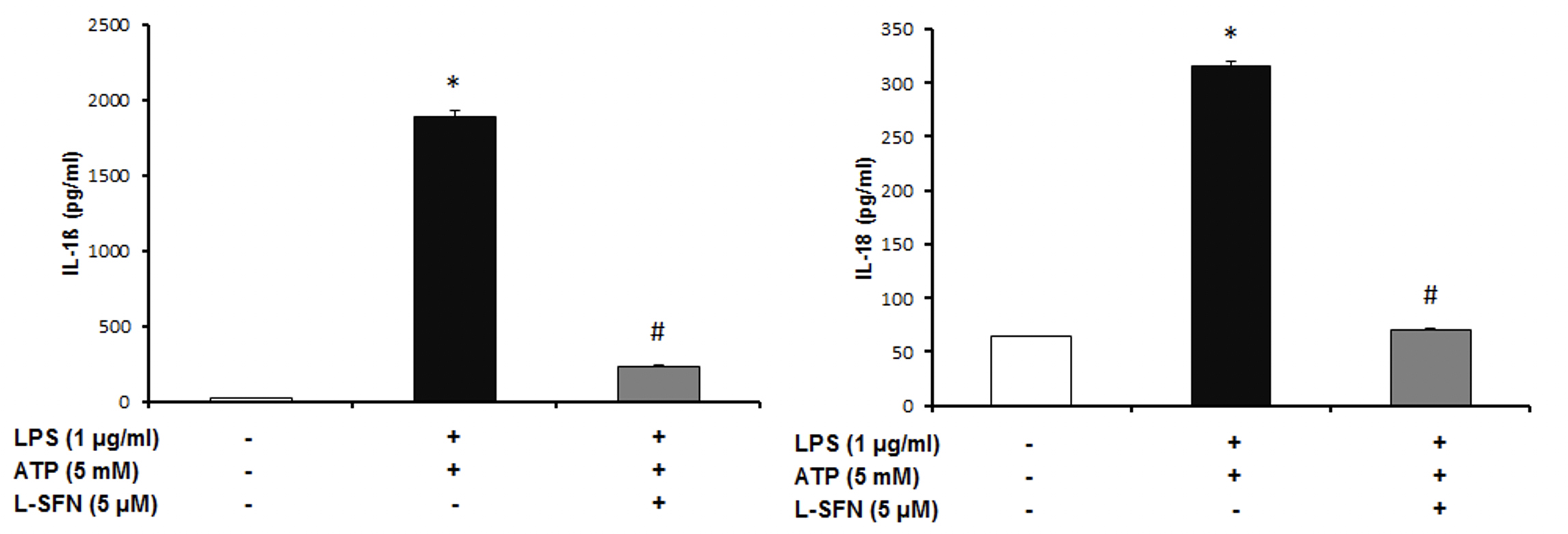
Figure 3: SFN significantly
After that, we asked whether sulforaphane is effective at
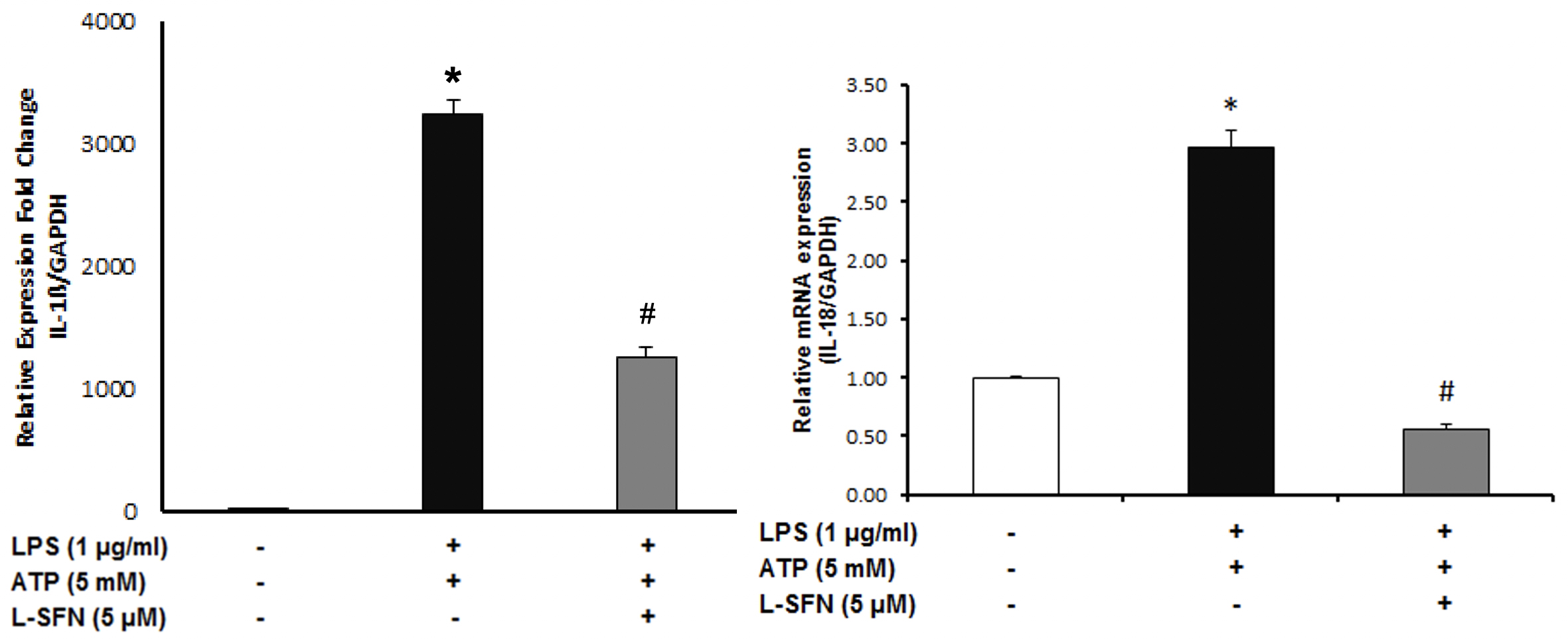
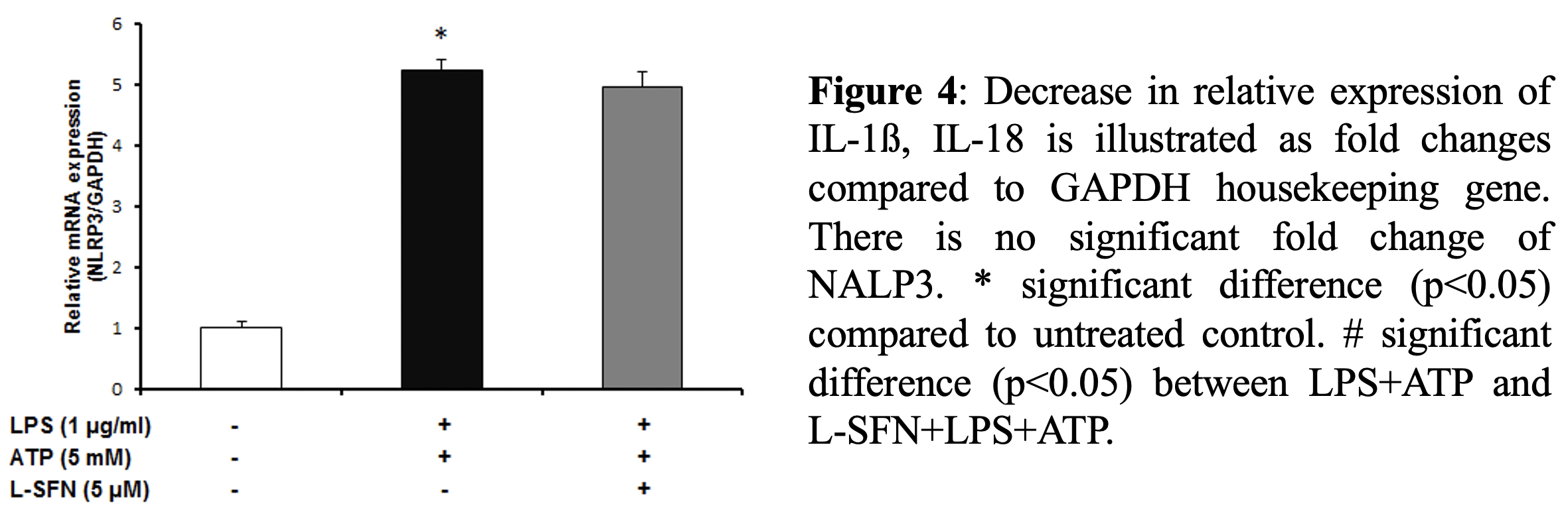
After mRNA level observations, we focused on the protein levels of major pro-inflammatory cytokine IL-1ß. To investigate
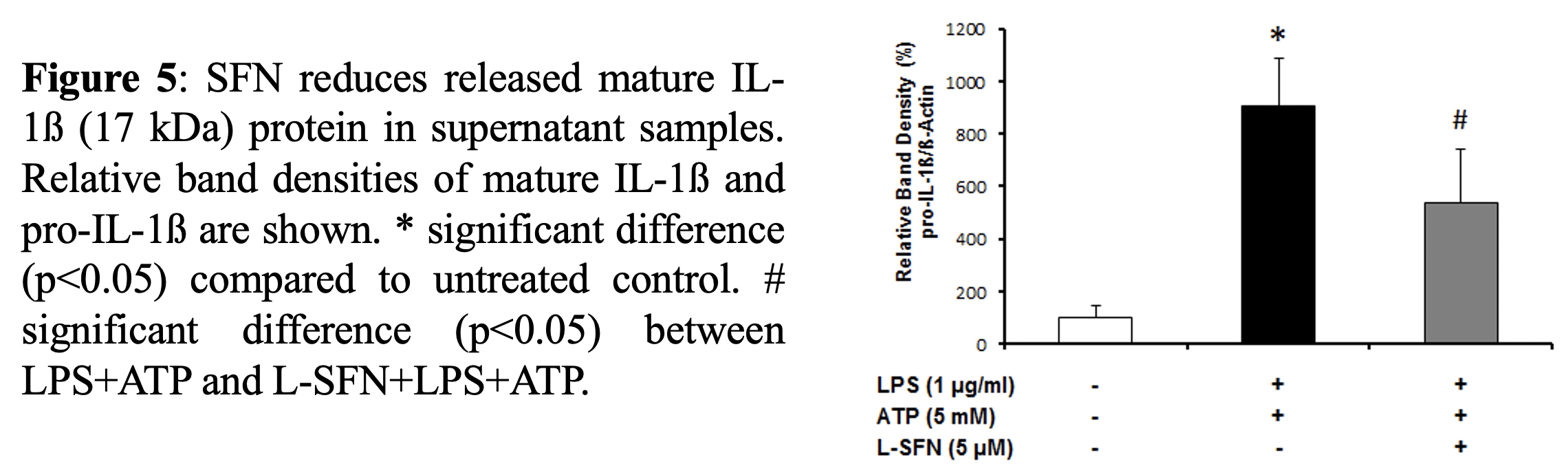
Conclusion:
In our recent study, we used
Source Paper: https://www.researchgate.net/publication/326736180_SULFORAPHANE_INHIBITS_NLRP3_INFLAMMASOME_ACTIVATION_IN_MURINE_MICROGLIAL_CELLS
Resources:
[1] Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, et al. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. 2009;57: 807–14
[2] Lawson L. J., Perry V. H., Gordon S. (1992). “Turnover of resident microglia in the normal adult mouse brain”. Neuroscience 48: 405–415
[3] Lamkanfi M. et al., 2009. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 284(31): 20574-81
[4] Pelegrin P, Surprenant A., 2007. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 282(4):2386-94.
